



Media
Good News: Hua Medicine’s Global First-in-Class Drug Was Presented at 2019 Scientific Meeting of CDS



November 25, 2019, Shanghai
On November 21st, the 2019 CDS Meeting (the 23rd Scientific Meeting of the Chinese Diabetes Society) was held in Xiamen. The Phase III clinical study results of dorzagliatin (HMS5552), the global first dual-acting glucokinase activator, were presented in the plenary session.

Dalong Zhu, M.D., chairman of the meeting, current chairman of CDS
Professor Dalong Zhu, chairman of the meeting and current chairman of CDS, gave a keynote speech entitled"Focus on Frontier, Innovation Management, Focus on Prevention – Healthy China CDS in Action", which introduced the major achievements in the field of diabetes in China in the past year. As one of the important achievements in the field of diabetes in China in 2019, Professor Dalong Zhu introduced the latest Phase III clinical results of dorzagliatin (HMS5552), a global first-in-class drug, glucokinase activator independently developed by Hua Medicine.

Hua Medicine’s Global First-in-Class Drug was presented in the plenary session at 2019 Scientific Meeting of CDS
Professor Dalong Zhu introduced to all the participants: dorzagliatin achieved primary efficacy endpoint in the Phase III clinical study of monotherapy (HMM0301) in drug naïve type 2 diabetes patients, with good safety, tolerance and significant therapeutic effect. He believed that the results of this clinical study were very encouraging. Professor Ralph DeFronzo, an authoritative expert on international diabetes, also made a special trip to Shanghai to express his appreciation and congratulations on the clinical results of dorzagliatin and discussed the next plan of the combination treatment with other drugs. Professor Dalong Zhu also said that the Phase III combination with metformin trial of dorzagliatin (HMM0302) in the treatment of metformin-tolerated type 2 diabetes patients was ongoing in China.
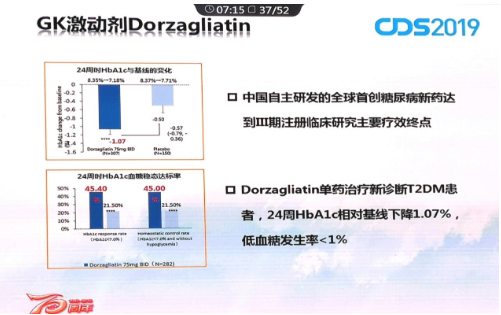
Great clinical performance of Dorzagliatin
In reality, global diabetes treatment faces enormous challenges. The number of people with diabetes is huge and the task of prevention and treatment is arduous. Professor Dalong Zhu pointed out that the number of patients with diabetes in China has reached 114 million and the incidence rate is as high as 10% to 11%. However, the awareness rate is only 38.6% and the treatment rate is 35.6%. Although it has improved significantly in recent years, the current status of Chinese diabetes treatment is still not optimistic.
The reason behind this is that existing drugs cannot address the root of the problem - "reduced glucose sensitivity" and these drugs can not effectively control the occurrence and development of diabetes, leading to a degenerative chronic disease where islet function (or also known as beta cell function) continues to deteriorate.
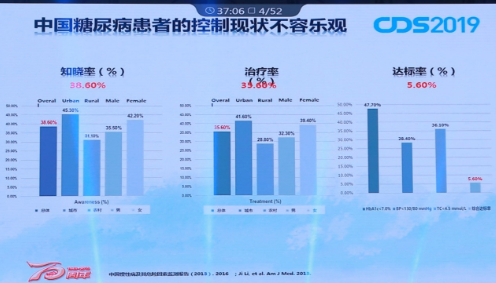
The current status of Chinese diabetic patients is not optimistic
Based on the global leading scientific theory of glucose homeostasis and the role of glucokinase as a glucose sensor in its regulation, Hua Medicine has developed a new diabetes drug—dorzagliatin. Clinical and basic research indicates that dorzagliatin can effectively improve the function of glucokinase and the sensitivity of human body to glucose to treat the root cause of diabetes.
The scientists of Hua Medicine have taken the lead in exploring the two-way regulation of glucokinase on blood glucose, repairing the loss of regulation of blood glucose in patients with diabetes to make dorzagliatin not only lower blood sugar, but also improve islet function and maintain blood sugar in a normal range to avoid the occurrence of hypoglycemia. According to the phase III study results, the incidence of clinically relevant hypoglycemia in dorzagliatin treated group was less than 1% and no severe hypoglycemia occurred. In the early study of the mechanism of action, it was verified that dorzagliatin improved the function of islet cells and insulin resistance.

Dr. Yi Zhang, project designer and manager of 301, senior vice president of Hua Medicine, said with confidence: "Dorzagliatin's Phase III registration clinical study results being presented at the CDS meeting is the result of the continuous improvement in the research and development of new diabetes drugs in China, which indicates the ability and strength of independent research and development of new Chinese medicines and this is the pride of all the Chinese clinical experts and scientists who are working hard on the dorzagliatin project."
This is undoubtedly an important milestone in the development of Hua Medicine and dorzagliatin. The development of the global first-in-class drug has become an important part of prevention and control of diabetes in China and the “Healthy China” program, which will continue to encourage Hua Medicine in “ For Patients, Global Innovation, Effective Medicines" on the road ahead with high standards, high quality and to create high value for global diabetes patients.

In addition, Professor Dalong Zhu also introduced the "Blue Light Action" initiated by the CDS. This year's "Blue Light Project" has expanded from "Blue Light Week" to "Blue Light Month" which means the Blue Light Action continues to raise awareness of the prevention and control of diabetes over a month-long period amongst the government, society and every family. “Implementing lifestyle intervention to the national chronic disease management level and exploring the sustainable development path for long-term management of Chinese diabetes patients,” he said, “strengthening lifestyle interventions and clinical standardized treatment is likely to be the way out for future diabetes treatment in China.”
Dr. Li Chen, the founder and CEO of Hua Medicine, concurred and said that diabetes management is not just drug treatment, but includes five ‘carriages’ —controlling diet, strengthening exercise, self-monitoring, diabetes education and drug treatment. In Hua Medicine’s clinical trials, clinical researchers strictly implement the diet and exercise management plan based on the guidance of the Chinese Diabetes and Endocrine Society, adhere to the education management of diabetes patients and provide a positive and meaningful practice for comprehensive treatment for diabetes and improving the health status of patients in China.
About Dorzagliatin
Dorzagliatin is a first-in-class, dual-acting glucokinase activator, designed to control the progressive degenerative nature of diabetes by restoring glucose homeostasis in people with type 2 diabetes. By addressing the defect of the glucose sensor function of glucokinase, dorzagliatin has the potential to restore the impaired glucose homeostasis state of people with type 2 diabetes and serve as a first-line standard-of-care therapy for the treatment of the disease, or as a cornerstone therapy when taken in combination with currently approved anti-diabetes drugs.
About Hua Medicine
Hua is a leading, clinical-stage innovative drug development company in China focused on developing novel therapies for the treatment of diabetes. Founded by an experienced group of entrepreneurs and international investment firms, Hua advanced a first-in-class oral drug for the treatment of type 2 diabetes into NDA-enabling stage and is currently evaluating the therapy in adults with diabetes in two Phase III trials in China and in two Phase I trials in the United States. The company has also initiated product life-cycle management studies of this novel diabetes therapy and advanced its use in personalized diabetes care. Hua Medicine's strategy is to leverage the cost-efficient and high-quality drug development capabilities available in China, while working closely with disease experts and regulatory agencies in China and across the world to advance diabetes care solutions for patients worldwide.
For more information
Hua Medicine
Email: ir@ytrongmao.com
Website: www.ytrongmao.com



沪ICP备14036654号-1
 沪公网安备 31011502013809号 Privacy Statement Terms of Use
沪公网安备 31011502013809号 Privacy Statement Terms of Use













